Central nervous system, or CNS, infections can be life-threatening if they are not diagnosed and treated early. Because CNS infections are non-specific, determining an accurate diagnosis can be challenging. The nucleic acid in vitro amplification-based molecular methods are starting to be utilized for routine microbial diagnosis. These molecular methods have improved beyond conventional diagnostic techniques with increased sensitivity and specificity. Moreover, molecular methods utilized on cerebrospinal fluid samples are considered the new standard for diagnosis of CNS infections caused by pathogens.
Molecular methods for the diagnosis of CNS infections offers a variety of monoplex and multiplex PCR assays to diagnose several types of health issues. Pan-omic molecular platforms can also help diagnose CNS infections. Although molecular methods are utilized for the diagnosis of CNS infections, the outcome measures for these diagnostic techniques must be carefully identified by healthcare professionals. The following article discusses conventional diagnostic techniques and molecular methods utilized for the diagnosis of central nervous system infections, their application, and future approaches.
Table of Contents
Molecular Methods in the Diagnosis of CNS Infections
Because of increased sensitivity and specificity, nucleic acid in vitro amplification-based molecular methods has tremendously improved the ability to diagnose CNS infections in a reasonable and effective time frame. Several PCR-derived techniques have also ultimately increased the flexibility and rigor of currently available diagnostic techniques.
Reverse transcriptase, or RT,-PCR was developed to increase RNA targets. Its utilization plays a fundamental role in the diagnosis of RNA-virus infections as well as managing their reaction to treatment. Timely access to enterovirus RT-PCR outcome measures has demonstrated shorter hospital stays, reduced unnecessary antibiotic utilization, and decreased ancillary laboratory evaluations and tests. Broad-range rRNA PCR techniques, which utilize a single pair of primers targeting conserved regions of genes, have been utilized to diagnose bacterial pathogens and herpes viruses in the CSF. Isothermal amplification-based techniques. including loop-mediated isothermal amplification or LAMP, have been developed to offer a diagnosis within several minutes to hours. Table 2 demonstrates commercial molecular in vitro diagnostic devices, or IVD, which have been cleared by the US Food and Drug Administration, or FDA, for diagnosis of microbial pathogens in CSF.
Monoplex Assays
A conventional molecular method involves three phases: sample extraction, target nucleic acid amplification, and amplicon detection. One of the first molecular assays successfully utilized for the diagnosis of CNS infections was utilized for the diagnosis of HSV in cerebrospinal fluid or CSF. PCR became the test of choice when research studies demonstrated that CSF PCR was similar to culture of brain tissue for diagnosis of HSV encephalitis and meningitis. Many PCR based methods for the diagnosis of herpes and enteroviruses have become available with increased sensitivity compared to viral culture.
Real-time PCR with nucleic acid amplification and amplicon detection further improved the transition to molecular methods in clinical laboratories. Unlike conventional PCR, the real-time system is a “closed” system and it overcomes the fundamental problem of carryover contamination. At the time of manuscript preparation, three molecular assays utilized to help diagnose HSV and enteroviruses in CSF have ultimately been approved by the FDA as demonstrated in Table 2 of the previous article. Real-time PCR-based methods are the main diagnostic technique utilized to help diagnose the Zika virus, which was first reported in Uganda in 1947, and is now a worldwide concern after the virus spread widely in Brazil and Central America. Research studies developed a one-step RT-PCR assay utilized to diagnose the Zika virus in human serum with a limited detection of 7.7pfu/reaction. Along with plasma, the Zika virus RNA can be diagnosed through urine and plasma within the first 2 weeks after symptoms have manifested. In March 2016, the FDA approved a trioplex-PCR assay under emergency use authorization for the simultaneous diagnosis of Zika, Chikungunya, and Dengue viruses in serum, urine, CSF and amniotic fluid. The RT-PCR assay utilizes dual labeled hydrolysis probes with a LOD of 1.54×10 4 GCE/ ml of Zika virus in serum.
Introduction of real-time PCR based diagnostic assays have affected early and effective diagnosis of several bacterial infections. Isothermal amplification-based molecular assays have excellent performance characteristics and they don’t require any specialized equipment. These assays are fundamental for the utilization of on or near point-of-care testing. LAMP-based methods have been utilized to diagnose Neisseria meningitis, Streptococcus pneumoniae, Haemophilus influenzae type b, M. tuberculosis, and JEV in the CSF. The Xpert MTB/RIF assay has tremendously improved regulation of tuberculosis by offering an integrated and automated system which allows quick clinical decision making in a POC or near-care context. Several research studies have utilized the Xpert MTB/RIF to evaluate the diagnosis of M. tuberculosis in CSF from TB meningitis. In a meta-analysis of thirteen research studies, the pooled sensitivity of the Xpert assay was 80.5 percent, or 95 percent CI 59.0 percent to 92.2 percent, against culture and 62.8 percent, or 95 percent CI 47.7 percent to 75.8 percent, against composite standard. Utilizing a large volume of sample, of at least 8–10 ml, is necessary for testing CSF and centrifugation can cause considerable improvements in yield. Despite the lack of standardization for sample processing, WHO has allowed testing CSF with the automated Xpert MTB/RIF assay as the first-line test over conventional microscopy.
Multiplex Assays
Simplicity makes multiplex molecular assays fundamental for the diagnosis of a panel of microbial targets. Several multiplex PCR assays have been developed to diagnose bacterial pathogens in CSF targeting the most common causes of meningitis: S. pneumoniae, N. meningitis, H. influenzae, L. monocytogenes, S. agalactiae, S. aureus, E. coli, and M. pneumoniae. A multiplex PCR followed by Luminex suspension array can simultaneously diagnose eight bacterial and viral pathogens in CSF, including N. meningitis, S. pueumoniae, E. coli, S. aureus, L. monocytogenes, S. agalactiae, HSV-1/2, and VZV, among others.
Considering the variety of pathogens involved in CNS infection, application of comprehensive molecular panels with multiple bacterial and viral targets have improved the efficiency of diagnosis. The BioFire FilmArray Meningitis/Encephalitis panel is currently the only FDA cleared multiplex assay utilized for the diagnosis of six bacterial, such as Escherichia coli K1, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitides, Streptococcus agalactiae and Streptococcus pneumoniae, seven viral, such as cytomegalovirus, enterovirus, HSV-1, HSV-2, human herpesvirus 6 or HHV-6, human parechovirus and VZV, as well as a single fungal, such as Cryptococcus neoformans/gattii, target in CSF as demonstrated in Table 2. The integrated FilmArray system takes about an hour, with only 2 minutes of hands-on time. During the preparation of the manuscript, two research studies demonstrated the performance of this assay. Utilizing 48 samples from gram stain negative CSF samples from suspected cases of meningitis, research studies demonstrated that this system diagnosed more viral pathogens, such as EBV. Four cases of WNV and a single case of Histoplasma were not diagnosed by this assay. Among HIV infected patients in Uganda, the test performance demonstrated increased sensitivity and specificity for the diagnosis of Cryptococcus. Although the FilmArray Meningitis/Encephalitis panel offers a quick diagnosis of CNS infections, further research studies are needed to determine its performance for a variety of targets and other high-risk populations.
Co-infections are frequently found among immunocompromised patients and can ultimately be challenging to diagnose for clinicians. The multiplex design allows simultaneous diagnosis of multiple targets on the same sample. One research study utilized a panel of monoplex and multiplex molecular assays to conduct a prospective cohort research study in Uganda to comprehensively evaluate the etiology of meningitis among HIV-infected adults. Among the 314 HIV-infected patients with meningitis, EBV co-infection was diagnosed with Cryptococcus, M. tuberculosis, or other viral pathogens. EBV in CSF in these settings is not completely understood although a single research study associated increased EBV viral load as a marker of poor outcome measures in patients with bacterial meningitis and EBV co-infection/ reactivation, among others.
Pan-Omic Molecular Assays
Technological improvements in metagenomic deep sequencing have increased its utilization for clinical diagnosis of CNS infections. Several research studies have demonstrated its ability to solve diagnostic technique problems which challenge the limits of traditional laboratory testing. Due to sterile status and protection by BBB, CSF and brain biopsies are fundamental to further explore the utilization of this technology for pathogen diagnosis. Metagenomics was successfully utilized to establish a diagnosis of neuroleptospirosis in a 14-year-old boy with severe combined immunodeficiency who also suffered from recurrent bouts of fever, headache, and coma. Similarly, high-throughput RNA sequencing performed on brain biopsy from an 18-month-old boy with encephalopathy diagnosed a new Astrovirus as the cause. Despite the utilization of metagenomics for the diagnosis of infectious disease, there are many technological and practical concerns which need to be addressed before this form of diagnostic testing can become mainstream and part of the clinical standard of care.
Other promising advances have occurred in transcriptomics, proteomics and metabolomics. Host and microbial microRNA or miRNA, profiles have been utilized for a variety of inflammatory and infectious diseases. Two miRNAs, miR-155 and miRNA-29b, were reported as potential biomarkers for JEV infection and treatment targets for anti-JEV therapy. Host neural epidermal growth factor, including 2 and apolipoprotein B in CSF, was able to diagnose tuberculous meningitis with 83.3 percent to 89.3 percent sensitivity and 75 percent to 92 percent specificity. CSF metabolite profiling has been reported to be useful in the classification, diagnosis, epidemiology, and treatment assessment of CNS infections in HIV patients. CSF metabolic profile analysis demonstrated bioenergetic adaptation in regulating shifts of HIV-infected patients.
Outcome Measures Associated with Diseases
Diagnosis of an etiologic agent in patients with CNS infections needs consideration of the most common causative organisms, the available diagnostic techniques and molecular methods for these agents, and the highest-yield clinical specimens for evaluation and testing. Knowledge of the epidemiology and clinical presentation of specific agents is fundamental in selecting which diagnostic methods are appropriate for patients. Animal or vector exposures, geographic location, recent travel history, season of the year, exposure of ill contacts, and occupational exposures should be considered.
When selecting appropriate pathogen-specific molecular diagnostic methods, the following factors should be considered. CSF is the optimal specimen for PCR testing for patients with meningitis or meningoencephalitis. While indirect evidence can be determined by testing other specimen types, attempts should be made to obtain CSF samples early before treatment can compromise yield. Time of testing from the manifestation of symptoms is fundamental to understand and rule out false-negative results and recommend retesting within a certain time frame. By way of instance, HSV PCR can commonly render false-negative results if CSF sample is obtained very early or late in the process of HSE infection. Host health is also known to affect test performance characteristics. Immunocompromised patients are at risk for infection by a variety of opportunistic pathogens, by way of instance HHV-6, JC virus, Toxoplasma encephalitis in bone marrow transplant recipients and patients with HIV. Often, infection can be more severe, such as WNV, and challenging to diagnose in this population. Table 3 below demonstrates practical recommendations on application and pitfalls of molecular test for the diagnosis of CNS infections.
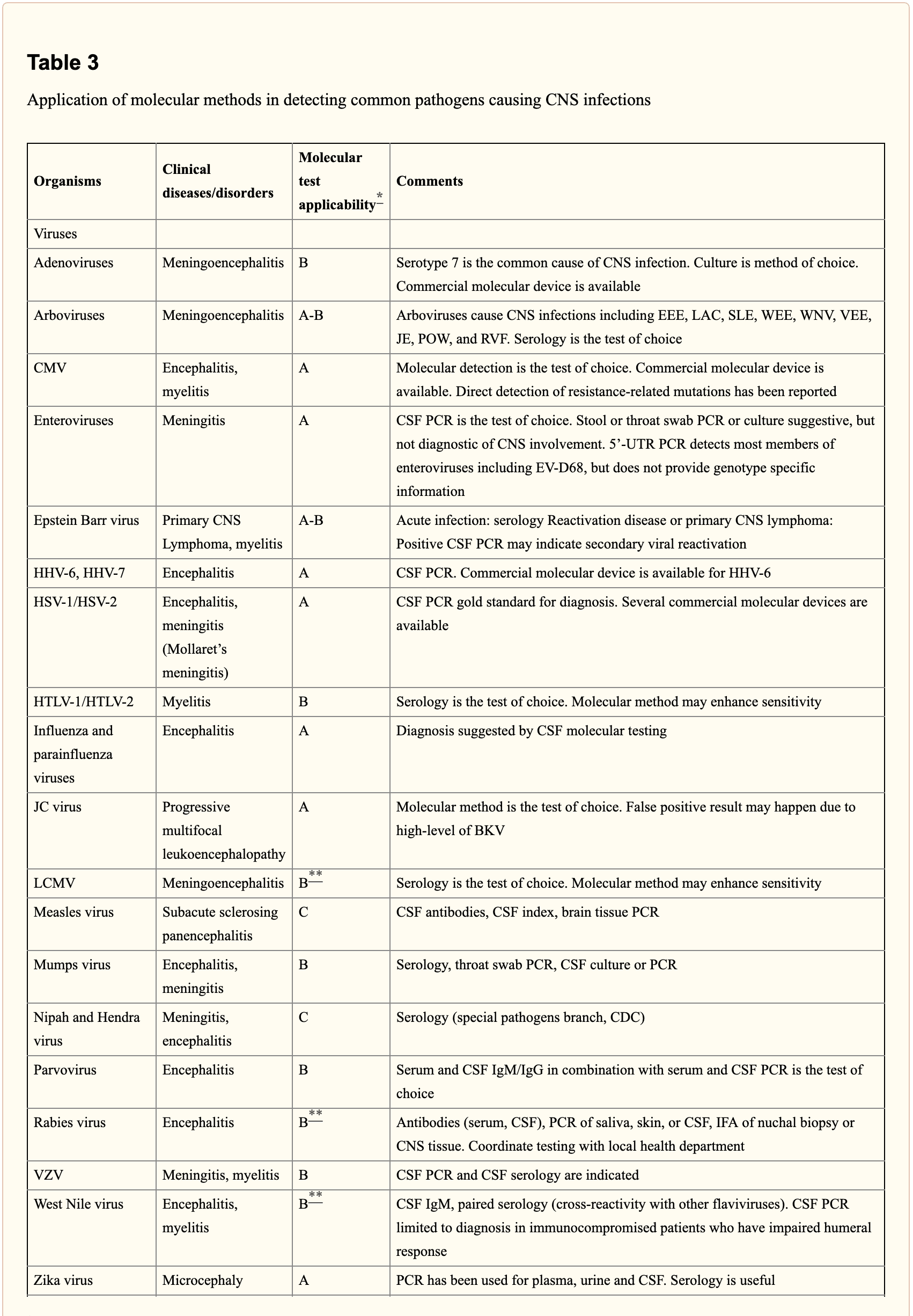
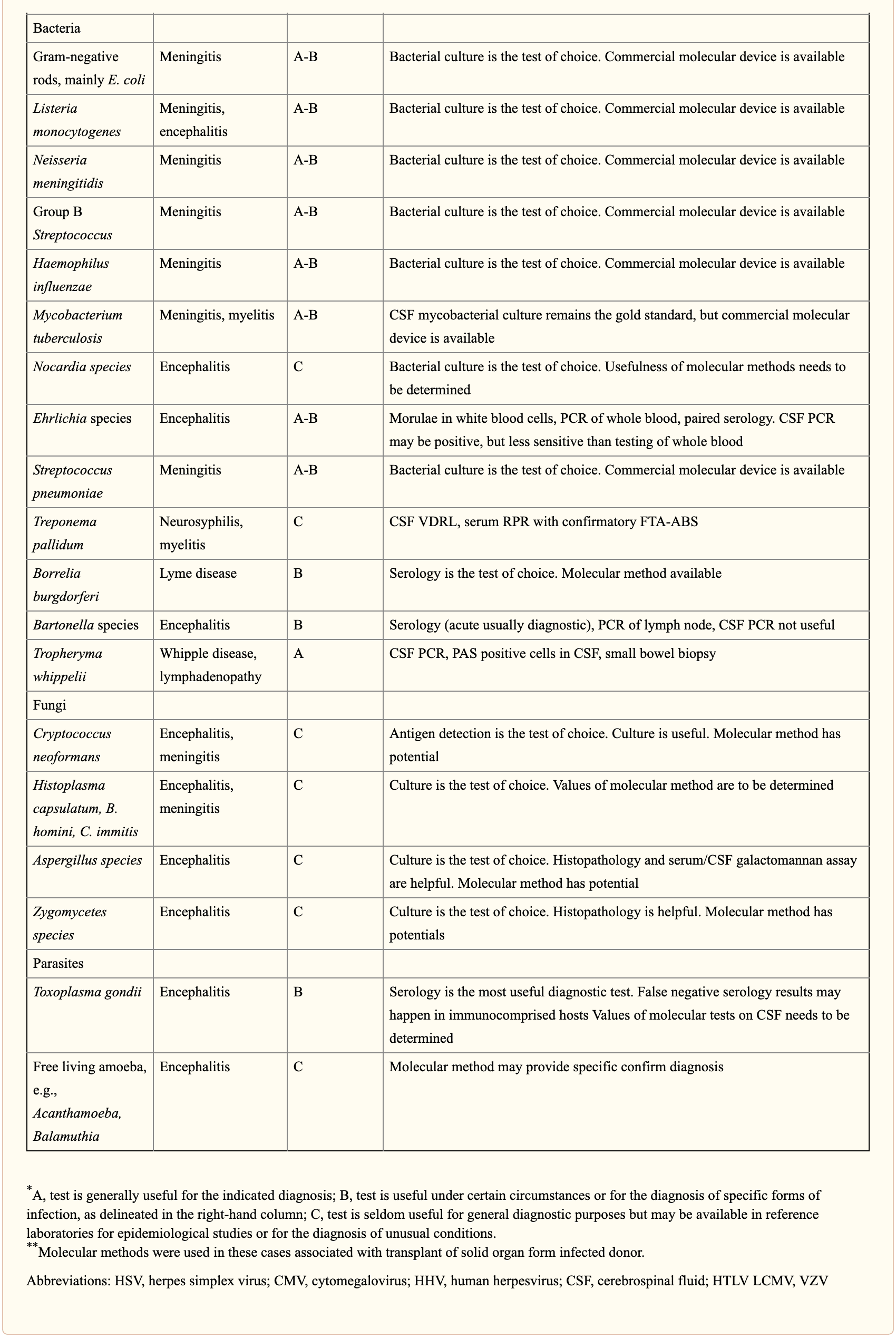
Furthermore, a positive nucleic acid amplification testing results are considered to be complicated by the fact that some viruses survive latently in macrophages or neurologic tissues even if they’re incidentally diagnosed by sensitive molecular techniques without an actual pathogenic role which can potentially lead to overtreatment. Utilization of adjunctive biomarkers which help determine active replication is being explored to overcome this drawback in research studies.
Historically, the diagnosis of microbiologic agents in patients with CNS infections has been hindered by the low yield of CSF culture for viral and fastidious bacterial organisms, delays in CNS production of organism-specific antibodies, and challenges in determining optimum samples for testing. The nucleic acid in vitro amplification-based molecular diagnostic methods and techniques have a wider and better application in clinical microbiology practice. The monoplex assay will likely be the main platform utilized for urgent, random-access, low throughput assays. Multiplex assays have the additional benefit of diagnosing multiple targets and mixed infections. As the volume of CSF sample retrieved is often small, multiplex assays enable comprehensive diagnostic analysis with a low amount of sample, obviating the need for repeated lumbar punctures. The clinical relevance and cost-effectiveness of simultaneous multi-pathogen diagnosis strategies need further research studies. Application of pan-omic techniques in challenging to diagnose CNS infections is the new exciting frontier, the technology is promising but routine implementation is expected to be slow due to various challenges, such as lack of applicable regulatory guidelines and adaptation in the clinical setting, although the outcome measures are promising.

As previously mentioned, central nervous system, or CNS, infections can be life-threatening health issues if they are not accurately diagnosed and properly treated. However, determining a diagnosis of CNS infections can be challenging for many clinicians. Fortunately, a variety of diagnostic techniques and molecular methods can ultimately help determine the source of CNS infections and other health issues. These diagnostic techniques and molecular methods have tremendously improved over the years, as previously mentioned, and more of these evaluations are being utilized in clinical settings to accurately diagnose CNS infections for proper treatment. – Dr. Alex Jimenez D.C., C.C.S.T. Insight
Diet and Exercise for Neurological Disease
In part 2 of our “Diagnosis of Central Nervous System Infections” article, we discussed the molecular methods and the pan-omic molecular assays which are utilized in the diagnosis of CNS infections as well as how specific testing outcome measures have ultimately been associated with clinical diseases and health issues. The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
Curated by Dr. Alex Jimenez
Additional Topic Discussion: Chronic Pain
Sudden pain is a natural response of the nervous system which helps to demonstrate possible injury. By way of instance, pain signals travel from an injured region through the nerves and spinal cord to the brain. Pain is generally less severe as the injury heals, however, chronic pain is different than the average type of pain. With chronic pain, the human body will continue sending pain signals to the brain, regardless if the injury has healed. Chronic pain can last for several weeks to even several years. Chronic pain can tremendously affect a patient’s mobility and it can reduce flexibility, strength, and endurance.
Neural Zoomer Plus for Neurological Disease
Dr. Alex Jimenez utilizes a series of tests to help evaluate neurological diseases. The Neural ZoomerTM Plus is an array of neurological autoantibodies which offers specific antibody-to-antigen recognition. The Vibrant Neural ZoomerTM Plus is designed to assess an individual’s reactivity to 48 neurological antigens with connections to a variety of neurologically related diseases. The Vibrant Neural ZoomerTM Plus aims to reduce neurological conditions by empowering patients and physicians with a vital resource for early risk detection and an enhanced focus on personalized primary prevention.
Formulas for Methylation Support
XYMOGEN’s Exclusive Professional Formulas are available through select licensed health care professionals. The internet sale and discounting of XYMOGEN formulas are strictly prohibited.
Proudly, Dr. Alexander Jimenez makes XYMOGEN formulas available only to patients under our care.
Please call our office in order for us to assign a doctor consultation for immediate access.
If you are a patient of Injury Medical & Chiropractic Clinic, you may inquire about XYMOGEN by calling 915-850-0900.
For your convenience and review of the XYMOGEN products please review the following link.*XYMOGEN-Catalog-Download
* All of the above XYMOGEN policies remain strictly in force.
Post Disclaimer
Professional Scope of Practice *
The information on this blog site is not intended to replace a one-on-one relationship with a qualified healthcare professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness and Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those found on this site and our family practice-based chiromed.com site, focusing on restoring health naturally for patients of all ages.
Our areas of chiropractic practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Chronic Pain, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is limited to chiropractic, musculoskeletal, physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for the injuries or disorders of the musculoskeletal system.
Our videos, posts, topics, subjects, and insights cover clinical matters and issues that relate to and directly or indirectly support our clinical scope of practice.*
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies available to regulatory boards and the public upon request.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: coach@elpasofunctionalmedicine.com
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License # TX5807
New Mexico DC License # NM-DC2182
Licensed as a Registered Nurse (RN*) in Texas & Multistate
Texas RN License # 1191402
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card