Alessio Nencioni, Irene Caffa, Salvatore Cortellino
Abstract | The vulnerability of cancer cells to nutrient deprivation and their dependency on specific metabolites are emerging hallmarks of cancer. Fasting or fasting-mimicking diets (FMDs) lead to wide alterations in growth factors and in metabolite levels, generating environments that can reduce the capability of cancer cells to adapt and survive and thus improving the effects of cancer therapies. In addition, fasting or FMDs increase resistance to chemotherapy in normal but not cancer cells and promote regeneration in normal tissues, which could help prevent detrimental and potentially life-threatening side effects of treatments. While fasting is hardly tolerated by patients, both animal and clinical studies show that cycles of low-calorie FMDs are feasible and overall safe. Several clinical trials evaluating the effect of fasting or FMDs on treatment-emergent adverse events and on efficacy outcomes are ongoing. We propose that the combination of FMDs with chemotherapy, immunotherapy or other treatments represents a potentially promising strategy to increase treatment efficacy, prevent resistance acquisition and reduce side effects.
Dietary and lifestyle-related factors are key determinants of the risk of developing cancer, with certain cancers being more dependent on dietary habits than others1–
Even though in the past decade we have witnessed unprecedented changes and remarkable advances in cancer treatment14,15, there remains a crucial need for more effective and, possibly,
Fasting forces healthy cells to enter a slow division and highly protected mode that protects them against toxic insults derived from anticancer drugs while sensitizing different types of cancer cells to these therapeutics11,12,17. This discovery implies that a single dietary intervention could potentially help address different and equally important aspects of cancer therapy.
In this Opinion article, we discuss the biological rationale for using fasting or fasting-mimicking diets (FMDs) to blunt TEAEs but also to prevent and treat cancer. We also illustrate the caveats of this experimental approach18,19 and the published and ongoing clinical studies in which fasting or FMDs have been applied to patients with cancer.
Table of Contents
Systemic & Cellular Fasting Response
Fasting leads to changes in the activity of many metabolic pathways associated with the switch into a mode able to generate energy and metabolites using carbon sources released primarily from adipose tissue and in part from muscle. The changes in the levels of circulating hormones and metabolites translate into a reduction in cell division and metabolic activity of normal cells and ultimately protect them from chemotherapeutic insults11,12. Cancer cells, by disobeying the anti-growth orders dictated by these starvation conditions, can have the opposite response of normal cells and therefore become sensitized to chemotherapy and other cancer therapies.
Systemic Response To Fasting
The response to fasting is orchestrated in part by the circulating levels of glucose, insulin, glucagon, growth hormone (GH), IGF1, glucocorticoids
Glucagon and low levels of insulin also stimulate the breakdown of triglycerides (which are mostly stored in adipose tissue) into glycerol and free fatty acids. During fasting, most tissues utilize fatty acids for energy, while the brain relies on glucose and on ketone bodies produced by hepatocytes (ketone bodies can be produced from acetyl-CoA generated from fatty acid ?-oxidation or from ketogenic amino acids). In the ketogenic phase of fasting, ketone bodies reach concentrations in the millimolar range, typically starting after 2–3 days from the beginning of the fast. Together with fat-derived glycerol and amino acids, ketone bodies fuel gluconeogenesis, which maintains glucose levels at a concentration of approximately 4mM (70mg per dl), which is mostly utilized by the brain.
Glucocorticoids and adrenaline also contribute to direct the metabolic adaptations to
Finally, fasting decreases the levels of circulating leptin, a hormone predominantly made by adipocytes that inhibits hunger, while increasing the levels of adiponectin, which increases fatty acid breakdown23,24. Thus, in conclusion, the hallmarks of the mammalian systemic response to fasting are low levels of glucose and insulin, high levels of glucagon and ketone bodies, low levels of IGF1 and leptin and high levels of adiponectin.
Cellular Response To Fasting
The response of healthy cells to fasting is evolutionarily conserved and confers cell protection, and at least in model organisms, has been shown to increase lifespan and healthspan12,22,25–31. The IGF1
Fasting and the resulting glucose restriction inhibit PKA activity, increase AMPK activity and activate EGR1 and thereby achieve cell-protective effects, including those in the myocardium22,25,26. Lastly, fasting and FMDs (see below for their composition) also have the ability to promote regenerative effects (Box 1) by molecular mechanisms, some of which have been implicated in cancer, such as increased autophagy or induction of sirtuin activity22,37–49.

Dietary Approaches In Cancer FMDs
The dietary approaches based on fasting that have been investigated more extensively in oncology, both preclinically and clinically, include water fasting (abstinence from all food and drinks except for water) and FMDs11,12,17,25,26,50–60 (Table 1). Preliminary clinical data indicate that a fast of at least 48hours may be required to achieve clinically meaningful effects in oncology, such as preventing chemotherapy-induced DNA damage to healthy tissues and helping to maintain
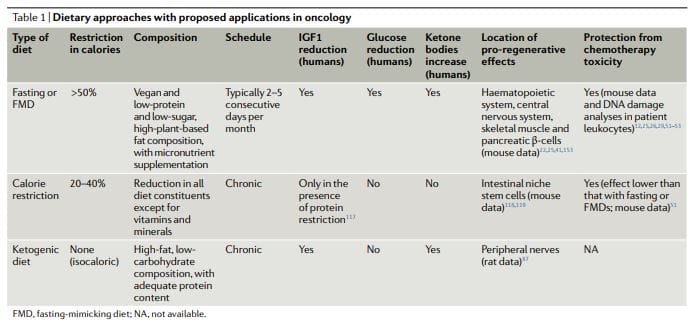
However, most patients refuse or have difficulties completing water fasting, and the potential risks of the extended calorie and micronutrient deficiency associated with it are difficult to justify. FMDs are medically designed dietary regimes very low in calories (that is, typically between 300 and 1,100kcal per day), sugars and proteins that recreate many of the effects of water-only fasting but with better patient compliance and reduced nutritional risk22,61,62. During an FMD, patients typically receive unrestricted amounts of water, small, standardized portions of vegetable broths, soups, juices, nut bars, and herbal teas, as well as supplements of micronutrients. In a clinical study of 3 monthly cycles of a 5-day FMD in generally healthy subjects, the diet was well tolerated and reduced trunk and total body fat, blood pressure and IGF1 levels62. In previous and ongoing oncological clinical trials, fasting or FMDs have typically been administered every 3–4 weeks, for example, in combination with chemotherapy regimens, and their duration has ranged between 1 and 5 days52,53,58,61,63–68. Importantly, no serious adverse events (level G3 or above, according to Common Terminology Criteria for Adverse Events) were reported in this studies52,53,58,61.
Ketogenic Diets
Ketogenic diets (KDs) are dietary regimens that have normal calorie, high-fat and low-carbohydrate content69,70. In a classical KD, the ratio between the weight of fat and the combined weight of carbohydrate and protein is 4:1. Of note, FMDs are also ketogenic because they have high-fat content and have the ability to induce substantial elevations (?0.5mmol per litre) in the levels of circulating ketone bodies. In humans, a KD can also reduce IGF1 and insulin levels (by more than 20% from baseline values), although these effects are affected by the levels and types of carbohydrates and protein in the diet71. KDs can reduce blood glucose levels, but they normally remain within the normal range (that is,>4.4mmol per litre)71.
Notably, KDs may be effective for preventing the increase in glucose and insulin that typically occurs in response to PI3K inhibitors, which was proposed to limit their efficacy72. Traditionally, KDs have been used for treating refractory epilepsy, mainly in children69. In mouse models, KDs induce anticancer effects, particularly in glioblastoma70,72–86. Clinical studies indicate that KDs probably have no substantial therapeutic activity when used as single agents in patients with cancer and suggest that potential benefits of these diets should be sought in combination with other approaches, such as chemotherapy, radiotherapy, antiangiogenic treatments, PI3K inhibitors
KDs were reported to have neuroprotective effects in peripheral nerves and in the hippocampus87,88. However, it remains to be established whether KDs also have proregenerative effects similar to fasting or FMDs (Box 1) and whether KDs also can be used to protect living mammals from the toxicity of chemotherapy. Notably, the regenerative effects of fasting or FMDs appear to be maximized by the switch from the starvation-response mode, which involves the breakdown of cellular components and the death of many cells, and the re-feeding period, in which cells and tissues undergo reconstruction22. Because KDs do not force entry into a starvation mode, do not promote a major breakdown of intracellular components and tissues and do not include a refeeding period, they are unlikely to cause the type of coordinated regeneration observed during the FMD refeeding.
Calorie Restriction
While chronic calorie restriction (CR) and diets deficient in specific amino acids are very different from periodic fasting, they share with fasting and FMDs a more or less selective restriction in nutrients, and they have anticancer effects81,89–112. CR typically involves a chronic 20–30% reduction in energy intake from the standard calorie intake that would allow an individual to maintain a normal weight113,114. It is very effective in reducing cardiovascular risk factors and cancer incidence in model organisms, including primates108,109,114.
However, CR can cause side effects, such as changes in physical appearance, increased cold sensitivity, reduced strength, menstrual irregularities, infertility, loss of libido, osteoporosis, slower wound healing, food obsession, irritability, and depression. In patients with cancer, there are substantial concerns that it may exacerbate malnutrition and that it will unavoidably cause excessive loss of lean body mass18,113–116. CR reduces fasting blood glucose levels, though they remain within the normal range114. In humans, chronic CR does not affect IGF1 levels unless a moderate protein restriction is also implemented117.
Studies show that by reducing mTORC1 signaling in Paneth cells, CR augments their stem cell function and that it also protects reserve intestinal stem cells from DNA damage118,119, but it is unknown whether pro-regenerative effects in other organs are also elicited by CR. Thus, the available data suggest that fasting and FMDs create a metabolic, regenerative and protective profile that is distinct and probably more potent than that elicited by a KD or CR.
Fasting & FMDs In Therapy: Effects on hormone and metabolite levels
Many of the changes in the levels of circulating hormones and metabolites that are typically observed in response to fasting have the capability to exert antitumour effects (that is, reduced levels of glucose, IGF1, insulin and leptin and increased levels of adiponectin)23,120,121 and/or to afford protection of healthy tissues from side effects (that is, reduced levels of IGF1 and glucose). Because ketone bodies can inhibit histone deacetylases (HDACs), the fasting-induced increase of ketone bodies may help slow tumor growth and promote differentiation through epigenetic mechanisms122.
However, the ketone body acetoacetate has been shown to accelerate, instead of reduce, the growth of certain tumors, such as melanomas with mutated BRAF123. Those changes for which there is the strongest evidence for a role in the beneficial effects of fasting and FMDs against cancer are the reductions in the levels of IGF1 and glucose. At the molecular level, fasting or an FMD reduces intracellular signaling cascades including IGF1R–AKT–mTOR–S6K and cAMP–PKA signaling, increases autophagy, helps normal cells withstand stress and promotes anticancer immunity25,29,56,124
Differential Stress Resistance: Increasing Chemotherapy Tolerability
Some yeast oncogene orthologues, such as Ras and Sch9 (functional orthologue of mammalian S6K), are able to decrease stress resistance in model organisms27,28. In addition, mutations that activate IGF1R, RAS, PI3KCA or AKT, or that inactivate PTEN, are present in the majority of human cancers10. Together, this led to the hypothesis that starvation would cause opposite effects in cancer versus normal cells in terms of their ability to withstand cell stressors, including chemotherapeutics. In other words, starvation can lead to
According to the DSR hypothesis, normal cells respond to starvation by downregulating proliferation associated and ribosome biogenesis and/or assembly genes, which forces cells to enter a self-maintenance mode and shields them from the damage caused by chemotherapy, radiotherapy and other toxic agents. By contrast, in cancer cells, this self-maintenance mode is prevented through oncogenic changes, which cause constitutive inhibition of stress response pathways12 (Fig. 1). Consistent with the DSR model, short-term starvation or the deletion of proto-oncogene
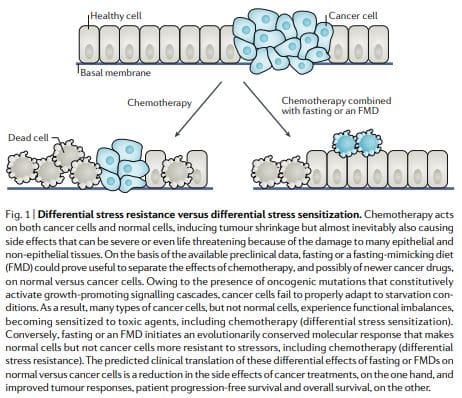
Similar results were obtained in mammalian cells: exposure to low-glucose media protected primary mouse glia cells against toxicity from hydrogen peroxide or cyclophosphamide (a prooxidant chemotherapeutic) but did not protect mouse, rat and human glioma and neuroblastoma cancer cell lines. Consistent with these observations,
Subsequent studies found that reduced IGF1 signaling in response to fasting protects primary glia and neurons, but not glioma and neuroblastoma cells, from cyclophosphamide and from pro-oxidative compounds and protects mouse embryonic fibroblasts from doxorubicin29. Liver IGF1-deficient (LID) mice, transgenic animals with a conditional liver Igf1 gene deletion that exhibit a 70–80% reduction in circulating IGF1 levels (levels similar to those achieved by a 72-hour fast in mice)29,125, were protected against three out of four chemotherapy drugs tested, including doxorubicin.
Histology studies showed signs of doxorubicin-induced cardiac myopathy in only doxorubicin-treated control mice but not in LID mice. In experiments with melanoma-bearing animals treated with doxorubicin, no difference in terms of disease progression between control and LID mice was observed, indicating that cancer cells were not protected from chemotherapy by reduced IGF1 levels. Yet, again, tumour-bearing LID mice exhibited a remarkable survival advantage compared with the control animals owing to their ability to withstand doxorubicin toxicity29. Thus, overall, these results confirmed that IGF1 downregulation is a key mechanism by which fasting increases chemotherapy tolerability.
Both dexamethasone and mTOR inhibitors are widely used in cancer treatment, either because of their efficacy as anti-emetics and
These interventions reduce PKA activity while increasing AMPK activity and thereby activating EGR1, indicating that cAMP– PKA signalling mediates the fasting-induced DSR via EGR1 (ref. 26). EGR1 also promotes the expression of cardioprotective peptides, such as the atrial natriuretic peptide (ANP) and the B-type natriuretic peptide (BNP) in heart tissue, which contributes to the resistance to doxorubicin. Furthermore, fasting and/or FMD might protect mice from doxorubicin-induced cardiomyopathy by boosting autophagy, which may promote cellular health by reducing reactive oxygen species (ROS) production through the elimination of dysfunctional mitochondria and by removal of toxic aggregates.
In addition to reducing chemotherapyinduced toxicity in cells and increasing survival of chemotherapy-treated mice, cycles of fasting induce bone marrow regeneration and prevent the immunosuppression caused by cyclophosphamide in a PKA-related and IGF1-related manner25. Thus, compelling preclinical results indicate the potential of fasting and FMDs to increase chemotherapy tolerability and to avoid major side effects. Because initial clinical data lend further support to this potential, these preclinical studies build a strong rationale for evaluating FMDs in randomized clinical trials with TEAEs as a primary end point.
Differential Stress Sensitization: Increasing The Death of Cancer Cells
If used alone, most dietary interventions, including fasting and FMDs, have limited effects against cancer progression. According to the differential stress sensitization (DSS) hypothesis, the combination of fasting or FMDs with a second treatment is much more promising11,12. This hypothesis predicts that, while cancer cells are able to adapt to limited oxygen and nutrient concentrations, many types of cancer cells are not able to execute changes that would allow survival in the nutrient-deficient and toxic environment generated by the combination of fasting and chemotherapy, for example. Early experiments in breast cancer, melanoma
We consider such an inappropriate response of cancer cells to the altered conditions including the reduction in IGF1 and glucose levels caused by fasting or FMDs as a key mechanism underlying the

By reducing glucose availability and increasing fatty acid ?-oxidation, fasting or FMDs can also promote a switch from aerobic glycolysis (Warburg effect) to mitochondrial oxidative phosphorylation in cancer cells, which is necessary for sustaining cancer cell growth in the most nutrient-poor environment50 (Fig. 2). This switch leads to increased ROS production11 as a result of increased mitochondrial respiratory activity and may also involve a reduction in cellular redox potential owing to decreased glutathione synthesis from glycolysis and the pentose phosphate pathway50. The combined effect of ROS augmentation and reduced antioxidant protection boosts oxidative stress in cancer cells and amplifies the activity of chemotherapeutics. Notably, because a high glycolytic activity demonstrated by high-lactate production is predictive of aggressiveness and metastatic propensity in several types of cancer129, the anti-Warburg effects of fasting or FMD have the potential to be particularly effective against aggressive and metastatic cancers.
Apart from a change in metabolism, fasting or FMDs elicit other changes that can promote DSS in pancreatic cancer cells. Fasting increases the expression levels of
Finally, fasting can upregulate the leptin receptor and its downstream
Notably, it is likely that many cancer cell types, including AML29, can acquire resistance by circumventing the metabolic changes imposed by fasting or FMDs, a possibility that is further increased by the metabolic heterogeneity that characterizes many cancers129. Thus, a major goal for the near future will be to identify the types of cancer that are most susceptible to these dietary regimens by means of biomarkers. On the other hand, when combined with standard therapies, fasting or FMDs have rarely resulted in the acquisition of resistance in cancer mouse models, and resistance to fasting combined with chemotherapy is also uncommon in studies in vitro, underlining the importance of identifying therapies that, when combined with FMDs, result in potent toxic effects against cancer cells with minimal toxicity to normal cells and tissues11,17,50,55–57,59,124.
Antitumour Immunity Enhancement by Fasting or FMD
Recent data suggest that fasting or FMDs by themselves, and to a greater extent when combined with chemotherapy, trigger the expansion of lymphoid progenitors and promote
Anticancer Diets in Mouse Models
Overall, the results of preclinical studies of fasting or FMDs in animal cancer models, including models for metastatic cancer (Table 2), show that periodic fasting or FMDs achieve pleiotropic anticancer effects and potentiate the activity of chemotherapeutics and TKIs while exerting protective and regenerative effects in multiple organs22,25. Achieving the same effects without fasting and/or FMDs would require first the identification and then the use of multiple effective, expensive and frequently toxic drugs and would probably be without the advantage of inducing healthy cell protection. It is noteworthy that in at least two studies fasting combined with chemotherapy proved to be the only intervention capable of achieving either complete tumour regressions or long-term survival in a consistent fraction of the treated animals11,59
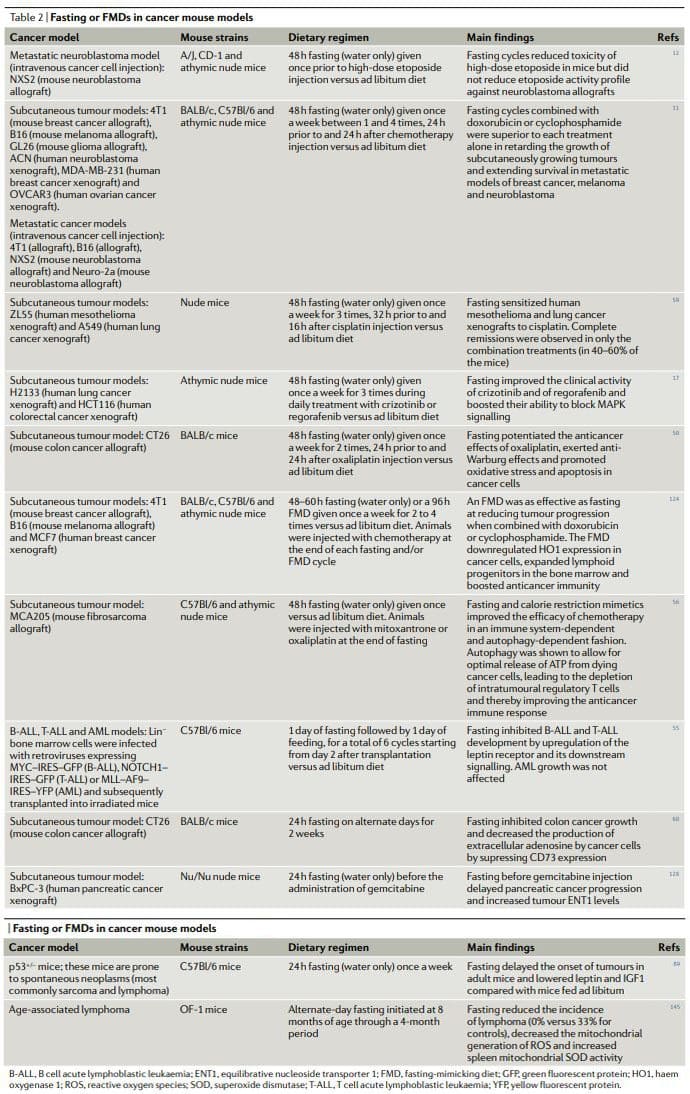
Chronic KDs also show a
CR reduced tumorigenesis in genetic mouse cancer models, mouse models with spontaneous tumorigenesis and carcinogen induced cancer mouse models, as well as in monkeys91,92,97,98,101,102,104–106,108,109,136–138. By contrast, a study found that CR from middle age actually increases the incidence of plasma cell neoplasms in C57Bl/6 mice139. However, in the same study, CR also extended maximum lifespan by approximately 15%, and the observed increase in cancer incidence was attributed to the increased longevity of mice undergoing CR, the age at which
Importantly, CR improved the activity of anticancer treatment in several cancer models, including the activity of an antiIGF1R antibody (ganitumab) against prostate cancer141, cyclophosphamide against neuroblastoma cells135 and autophagy inhibition in xenografts of HRAS-G12Vtransformed immortal baby mouse kidney epithelial cells100. However, CR or a KD in combination with anticancer therapies seems to be less effective than fasting. A mouse study found that, in contrast to fasting alone, CR alone was not able to reduce the growth of subcutaneously growing GL26 mouse gliomas and that, again, in contrast to short-term fasting, CR did not increase cisplatin activity against subcutaneous 4T1 breast tumours51. In the same study, fasting also proved substantially more effective than CR and a KD at increasing the tolerability of doxorubicin51. Although fasting or an FMD, CR and a KD likely act on and modulate overlapping
The phase of refeeding could then
Fasting and FMDs in Cancer Prevention
Epidemiological studies and studies in animals, including monkeys108,109,144, and humans lend support to the notion that chronic CR and periodic fasting and/or an FMD could have cancer-preventive effects in humans. Nevertheless, CR can hardly be implemented in the general population owing to issues of compliance and to possible side effects115. Thus, while evidence-based recommendations of foods to prefer (or to avoid) as well as lifestyle recommendations to reduce cancer risk are becoming established6,8,9,15, the goal now is to identify and, possibly, standardize well tolerated, periodic dietary regimens with low or no side effects and evaluate their cancer-preventive efficacy in clinical studies.
As discussed earlier, FMD cycles cause downregulation of IGF1 and glucose and upregulation of IGFBP1 and ketone bodies, which are changes similar to those caused by fasting itself and are biomarkers of the fasting response22. When C57Bl/6 mice (which spontaneously develop
A previous study of alternate-day fasting, which was performed in middle-aged mice for a total of 4 months, also found that fasting reduced the incidence of lymphoma, bringing it from 33% (for control mice) to 0% (in fasted animals)145, although because of the short duration of the study it is unknown whether this fasting regimen prevented or simply delayed the
Therefore, the promising results of preclinical studies combined with the clinical data on the effect of an FMD on risk factors for
Clinical Applicability in Oncology
Four feasibility studies of fasting and FMDs in patients undergoing chemotherapy have been published as of today52,53,58,61. In a case series of 10 patients diagnosed with various types of cancer, including breast, prostate, ovarian, uterus, lung and oesophageal cancer, who voluntarily fasted for up to 140hours before and/or up to 56hours following chemotherapy, no major side effects caused by fasting itself other than hunger and lightheadedness were reported58. Those patients (six) who underwent chemotherapy with and without fasting reported a significant reduction in fatigue, weakness and gastrointestinal adverse events while fasting. In addition, in those patients in which cancer progression could be assessed, fasting did not prevent chemotherapy-induced reductions in tumour volume or in tumour markers. In another study, 13 women with HER2 (also known as ERBB2) negative, stage II/III breast cancer receiving neo-adjuvant taxotere, adriamycin and cyclophosphamide (TAC) chemotherapy were randomized to fast (water only) 24hours before and after beginning chemotherapy or to nutrition according to standard guidelines52.
Short-term fasting was well tolerated and reduced the drop in mean erythrocyte and thrombocyte counts 7 days after chemotherapy. Interestingly, in this study, the levels of ?-H2AX (a marker of DNA damage) were increased 30minutes after chemotherapy in leukocytes from non-fasted patients but not in patients who had fasted. In a dose escalation of fasting in patients undergoing platinum-based chemotherapy, 20 patients (who were primarily treated for either urothelial, ovarian or breast cancer) were randomized to fast for 24, 48 or 72hours (divided as 48hours before chemotherapy and 24hours after chemotherapy)53. Feasibility criteria (defined as three or more out of six subjects in each cohort consuming?200kcal per day during the fast period without excess toxicity) were met. Fasting-related toxicities
Very recently, a randomized crossover clinical trial was conducted assessing the effects of an FMD on quality of life and side effects of chemotherapy in a total of 34 patients with breast or ovarian cancer61. The FMD consisted of
Challenges in The Clinic
The study of periodic fasting or of FMDs in oncology is not devoid of concerns, particularly in relation to the possibility that this type of dietary regimen could precipitate malnutrition, sarcopenia,
Conclusions
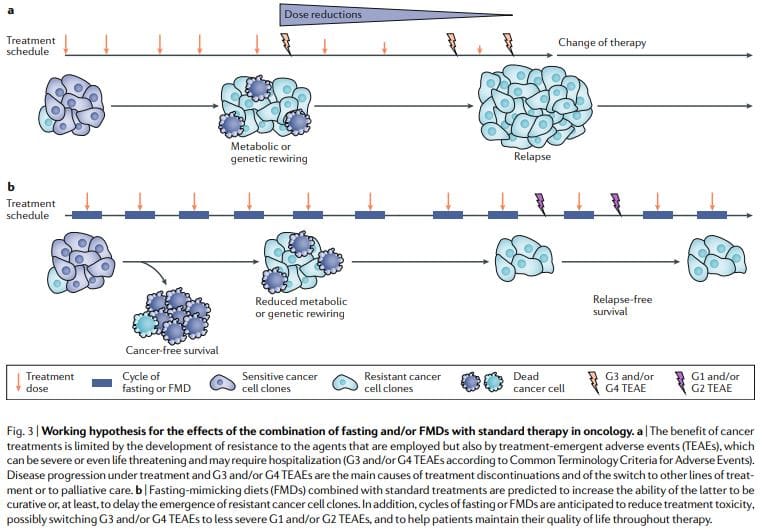
Periodic fasting or FMDs consistently show powerful anticancer effects in mouse cancer models including the ability to potentiate chemoradiotherapy and TKIs and to trigger anticancer immunity. FMD cycles are more feasible than chronic dietary regimens because they allow patients to consume food regularly during the FMD, maintain a normal diet between cycles and do not result in severe weight loss and possibly detrimental effects on the immune and endocrine systems. Notably, as standalone therapies, periodic fasting or FMD cycles would probably show limited efficacy against established tumors. In fact, in mice, fasting or FMDs affect the progression of a number of cancers similarly to chemotherapy, but alone, they rarely match the effect obtained in combination with cancer drugs which can result in cancer-free survival11,59. Thus, we propose that it is the combination of periodic FMD cycles with standard treatments that holds the highest potential to promote cancer-free survival in patients, as suggested by the mouse models11,59 (Fig. 3).
This combination may be particularly potent for several reasons: first, cancer drugs and other therapies can be effective, but a portion of patients do not respond because cancer cells adopt alternative metabolic strategies leading to survival. These alternative metabolic modes are much more difficult to sustain under fasting or FMD conditions because of the deficiencies or changes in glucose, certain amino acids, hormones, and growth factors, as well as in other unknown pathways leading to cell death. Second, fasting or FMDs can prevent or reduce resistance acquisition. Third, fasting or FMDs protect normal cells and organs from the side effects caused by a wide variety of cancer drugs. On the basis of preclinical and clinical evidence of feasibility, safety and efficacy (at reducing IGF1, visceral fat
Furthermore, it is essential to apply FMDs with an understanding of the mechanisms of action, since their potency
Ongoing clinical studies of FMDs in patients with cancer63,65–68 will provide more solid answers as to whether prescribing periodic FMDs in combination with conventional anticancer agents helps improve tolerability and activity of the latter. It is important to consider that FMDs will not be effective in reducing the side effects of cancer treatments in all patients and neither will they work to improve the efficacy of all therapies, but they have great potential to do so at least for a portion and possibly for a major portion of patients and drugs. Frail or malnourished patients or patients at risk of malnutrition should not be enrolled in clinical studies of fasting or FMDs, and patient nutritional status and anorexia should be carefully monitored throughout clinical trials.
References:
Post Disclaimer
Professional Scope of Practice *
The information on this blog site is not intended to replace a one-on-one relationship with a qualified healthcare professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness and Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those found on this site and our family practice-based chiromed.com site, focusing on restoring health naturally for patients of all ages.
Our areas of chiropractic practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Chronic Pain, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is limited to chiropractic, musculoskeletal, physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for the injuries or disorders of the musculoskeletal system.
Our videos, posts, topics, subjects, and insights cover clinical matters and issues that relate to and directly or indirectly support our clinical scope of practice.*
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies available to regulatory boards and the public upon request.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: coach@elpasofunctionalmedicine.com
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License # TX5807
New Mexico DC License # NM-DC2182
Licensed as a Registered Nurse (RN*) in Texas & Multistate
Texas RN License # 1191402
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card